

Understanding Lubricant Physical Properties and Chemistry
Posted on 10/05/21 in: Product Knowledge | Technical Knowledge | Agriculture | Food & Beverage | Horizontal Directional Drilling | Military | Oilfield | Petrochemical | Oil & Gas | General Industry | Steel | Rail | Author: Mike Holloway
Lubricants have several physical properties that serve their function and performance.
- Viscosity
- Specific gravity and density
- Pour point
- Film strength
- Flashpoint
- Oxidation resistance
- Water separation
- Rust and corrosion protection
Viscosity
The most important property is viscosity. Viscosity, which measures oil’s resistance to flow, is the most important property of a lubricant. Water has a relatively low viscosity; molasses has a much higher viscosity. However, if you heated molasses, it would get thinner. Likewise, oils also get “thinner” as they get hot. Viscosity has an inverse relationship with temperature. As pressure increases, the viscosity of oil increases, too. Therefore, the viscosity of oil in service varies with its temperature and pressure.
The viscosity of industrial oils is generally reported at 40˚C. The International Standards Organization uses this as the standard for its ISO VG grading system that ranges from ISO VG 2 to ISO VG 1500. The ISO VG is defined as the midpoint of a range that is + 10%. For example, a hydraulic fluid with a viscosity of 31.5 cSt at 40C has an ISO VG of 32. The viscosity of crankcase oils is typically measured at 100C. Lubricating oils can range from very low viscosity like solvents and kerosene used for rolling metals, to high viscosity fluids that barely flow at room temperature, such as steam cylinder oils or gear oils used in sugar mills.
A characteristic of viscosity is the Viscosity Index. This is an empirical number that indicates the effect of change on the viscosity of a lubricant. A lubricant with high viscosity index does not thin down very fast as it heats up. It would be used for oils that are used outdoors in summer and winter. Multi-viscosity engine oils have a high viscosity index.
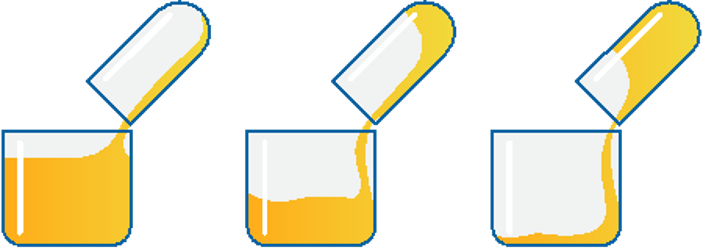
Specific Gravity and Density
Specific Gravity – the mass per unit volume of a substance is called density and is expressed in pounds per gallon, kg/m, or g/cc. The specific gravity is defined as the density of a substance divided by the density of water. A substance with a specific gravity greater than one is heavier than water and vice versa. It is a measure of how well a substance floats on top of water (or sinks below the surface.) Water has a density of approximately 1 g/cc at room temperature. Petroleum fluids generally have a specific gravity of less than 1, so they float. Oil slicks float on the surface of a puddle.
Water drains in reservoirs are positioned at the bottom of the reservoir. The lower the specific gravity, the better the oil floats. Oil with a specific gravity of 0.788 floats very well. The density of oils decreases with temperature; they float better as they heat up. Density of petroleum products is often expressed as API gravity which is defined as Degrees API = (141.5/ Sp Gravity @60˚F – 131.5). The API gravity of water is 10. Since API gravity is the reciprocal of specific gravity, the higher the API gravity, the lighter the oil; therefore the better it floats.
Pour Point
The Pour Point of oil is the lowest temperature at which it will pour, or flow, when chilled without disturbance. The very first additive that was used in engine oil was a Pour Point depressant additive.
Film Strength
Film strength is a measure of a fluid’s lubricity. It is the load-carrying capacity of a lubricant film. Film strength can be enhanced by the use of additives. Many synthetic oils have greater film strength than petroleum oils.
Flash Point
Flashpoint is the temperature at which the vapors of a petroleum fluid ignite when a small flame is passed over the surface. In order for combustion to occur, there has to be a certain air/fuel mixture. If there is too much air, the mixture is too lean – there’s not enough fuel. If there’s too much liquid, it essentially suffocates the flame.
The flashpoint is the temperature where there are enough molecules bouncing around in the air above the surface to produce an air/fuel mixture that will burn (if there is a spark to ignite them as evidenced by a popping sound.
The flashpoint is directly related to evaporation rate. A low viscosity fluid will generally evaporate faster than high viscosity oil, so its flash point is typically lower. For safety, it is a good idea to choose oil that has a flashpoint of at least 20°F higher than the highest operating temperature in the equipment. Fire point is the temperature that supports combustion for 5 seconds.
Oxidation Resistance
Oxidation resistance affects the life of the oil. Turbines and large circulating systems, in which oil is used for long periods without being changed, must have oils with high resistance to oxidation. Where oil remains in service only a short time or new oil is frequently added as make-up, those grades with lower oxidation resistance may serve satisfactorily.
The rate of oxidation of petroleum oils tends to double for every 18˚F (10°C) rise in temperature, therefore for every 18˚F(10°C) that you raise the temperature of a system, expect to change the oil twice as often. Another way of stating this is for every 18˚F decrease in oil temperature, oil life is doubled.
Water Separation
The separation of oil from water is called demulsibility. Water can cause rust, corrosion and wear, among many other detrimental factors such as foaming and cavitation. Some base oils have a natural repulsion to water whereas others are readily miscible. Certain additives can be used to offset the potential mixing which would lead to emulsification.
Circulating oil systems require oils that demulsify well. Once-through systems do not require demulsifiers because the oil doesn’t recirculate and collect enough water to cause rust. Demulsifiers are not necessary if the system is hot enough to boil off any water such as an engine. In certain instances, oil is mixed with water to improve fire retardancy or metalworking fluid cooling. Emulsions are important for fire resistance and metalworking cooling.
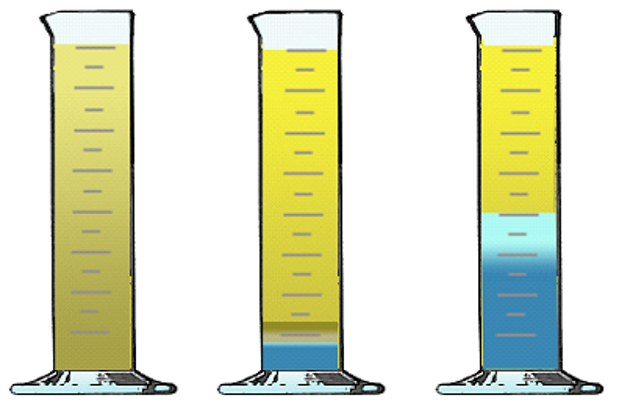
Water/Oil Mixture Partial Separation Full Separation
Rust and Corrosion Inhibiting
When machinery is idle, the lubricant may be called upon to act as a preservative. When machinery is in actual use, the lubricant controls corrosion by coating lubricated parts. Once at rest, the lubricant rust and corrosion inhibiting film has now coated the surface protecting it from water.
Lubricant Chemistry
Lubricants are built with a base oil(s) and additives. Petroleum oils account for most of the two general categories of industrial and transportation lubrication. They are refined from crude oil, which, as everyone knows, was formed from billions and billions of tiny microorganisms that converted over time and pressure to oil. The term hydrocarbon simply means that it is predominantly comprised of hydrogen and carbon, although there are small amounts of other elements such as sulfur and nitrogen.
The two principal types of petroleum oils used for lubricants are paraffinic and naphthenic. When you think of paraffin, you think of wax. That gives you a good idea of the strengths of paraffinic oil. Wax is an excellent lubricant; it is slippery and quite stable at high temperatures. It is ineffective at low temperatures because it turns solid. For this reason, paraffinic oils are recommended for most industrial and transportation lubricants, except where they run at cold temperatures. Another characteristic of wax is that it leaves very little residue when it oxidizes, but the small amount of residue is hard and sticky.

Naphthenic oils are not waxy, so they can be used to very low temperatures. While they tend to leave more deposits than paraffinic oil, what is left behind is soft and fluffy. Compressor manufacturers often prefer naphthenic oils because the deposits get blown out with the compressed air rather than building up on discharge valves. Naphthenic oils are also used in many refrigeration applications because of their good cold temperature properties.
Physically, paraffinic oils can be distinguished from naphthenic oils because of their higher pour points and lower density. Paraffinic oils typically weigh between 7.2 and 7.3 pounds per gallon, while naphthenic oils are slightly heavier. Be careful about characterizing the base stock of a formulated product based on physical properties because additives can strongly affect physical properties.
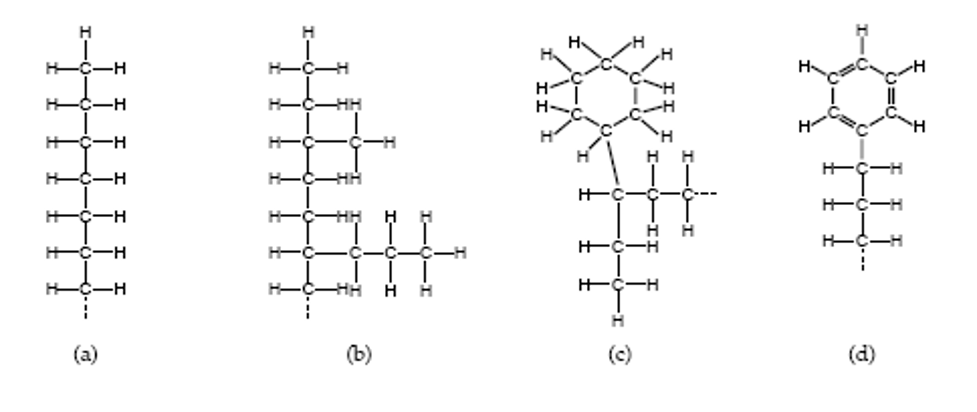
(a) and (b) - Paraffin, (c) - Naphthene, (d) - Aromatic
With the advent of more sophisticated refining techniques, base stocks have been categorized into Group I, Group II and Group III. Group I base stocks is conventionally refined oils. Group II is base stocks that contain greater than 90% saturates and less than .03% sulfur with a VI between 80-119. They are often produced by hydrocracking.
Base Oils | Satures Content | Sulfur Content | Viscosity Index |
Group I | <90 % | >0.03 % | 80 – 120 |
Group II | >90 % | <0.03 % | 80-120 |
Group III | >90 % | <0.03 % | >120 |
White oils are highly refined petroleum oils that meet food and drug requirements for direct food contact. Customers may ask that the product be certified as USDA H-1 for incidental food contact. While the USDA has disbanded the organization that tested and approved H-1 lubricants for incidental food contact, producers can now self-certify that their products were formally approved under H-1 or currently meet the requirements set forth by that standard.
Synthetic Base Oils
Synthetic base oils are produced, mainly, from low molecular weight hydrocarbons, the process produces high quality and extended service life capability base oils under extremes operating conditions. In general terms, synthetic base oils are able to handle a wider range of application temperatures, so they provide the best protection both to high and low temperatures.
[Text Wrapping Break]
Base Oils | Type of Base |
Group IV | Polyalphaolefin |
Group V | Other Synthetic Bases |
[Text Wrapping Break] API Classification (2nd part)
Synthetic Hydrocarbon Fluids
The SHFs comprise the fastest-growing type of synthetic lubricant base stock, they all are compatible with mineral base stocks.
Polyalphaolefins (PAO) are unsaturated hydrocarbons with the general formula (-CH2-)n, free of sulfur, phosphorus, metals and waxes. Provide excellent high-temperature stability and low-temperature fluidity, high viscosity indexes, low volatility and compatible with mineral base oils. Although the oxidation stability is lower than mineral oils and their solvency of polar additives is poor, usually PAOs are combined with other synthetic oils. This base oil is recommended for engine oils and gear oils.
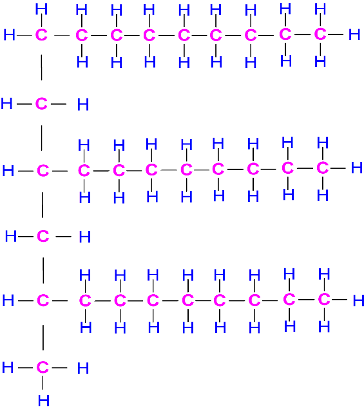
Alkylated Aromatics formed by alkylation of an aromatic compound, usually benzene or naphthalene. Provide excellent low-temperature fluidity and low pour points, good solubility for additives, thermal stability and lubricity. Although their viscosity index are about the same as mineral oils, they are less volatile, more stable to oxidation, high temperatures and hydrolysis. They are used as the base of engine oils, gear oils and hydraulic fluids.
Polybutenes are produced by controlled polymerization of butenes and isobutylenes. Compared with other synthetic base oils they are more volatile, less stable to oxidation and their viscosity index is lower; their tendency to produce smoke and shoot deposits is very low so they are used to formulate 2-Stroke engine oils, also as gear oils combined with mineral or synthetic base oils.
Polyalkylene Glycols (PAG) are polymers made from ethylene oxide (EO), propylene oxide (PO), or their derivatives. Solubility in water or other hydrocarbon is depending on the type of oxide. Both provide good viscosity/temperature characteristics, low pour point, high-temperature stability, high flash point, good lubricity, and good shear stability. PAGs are not corrosive for most metals and compatible with rubber. The main disadvantages are low additive solvency and pour compatibility with lubricants, seals, paints and finishes.
They are used as a base for hydraulic brake fluids (DOT3 and DOT 4) due to their water solubility, 2-Stroke engine oils due to the low deposits at high temperatures, compressor lubricants and fire-resistance fluids.
Synthetic Esters are oxygen-containing compounds that result from the reaction of an alcohol with an organic acid. They have good lubricity, temperature and hydrolytic stability, solvency of additives and compatibility with additives and other bases.
But some esters can damage seals so they require special compositions. They are used as base oils for engine oils, mixed with other synthetic bases, because they improve low-temperature properties, reduce fuel consumption, increase wear protection and viscosity-temperature properties.
Also, as 2-Stroke engine base oils, they reduce deposit formation, protecting rings, pistons and sparks. They allow you to reduce the quantity of lubricant from 50:1 of mineral oils to 100:1 and up 150:1 due to their outstanding lubricity.

Phosphate Esters are used as anti-wear additives due their high lubricity and as base oils for hydraulic fluids and compressor oils due to their low flammability. But their hydrolytic and temperature stability and viscosity index is low and their low-temperature properties are poor. Also, they are aggressive with paints, coats and seals.
Polyol Esters have good high-temperature stability, hydrolytic stability and low-temperature properties, low volatility and low Viscosity Index; the polyol esters also may have more effect on paints and cause more swelling of elastomers. To take advantage of their miscibility with hydrofluorocarbon (HFC) refrigerants, polyol esters are used in refrigeration systems.
Perfluorinated Polyethers (PFPE) with a density nearly twice that of hydrocarbons, they are immiscible with most of the other base oils and non-flammable under all practical condition. Very good viscosity-temperature and viscosity-pressure dependence, high oxidation and water stability, inert chemically and radiation stable; these properties joined their shearing stability. They are suitable as hydraulic fluids in spacecraft and as dielectric in transformers and generators.
Polyphenyl Ethers have excellent high-temperature properties and resistance to oxidation but they have fair viscosity-temperature properties, they are used as hydraulic fluid for high temperature and radiation resistance.
Polysiloxanes or Silicones have high viscosity index, over 300, low pour point, high-temperature stability and oxidation stability so they run well in a wide range of temperatures; they are chemically inert, non-toxic, fire-resistant, and water repellent, they have low volatility and are compatible with seals and plastics.
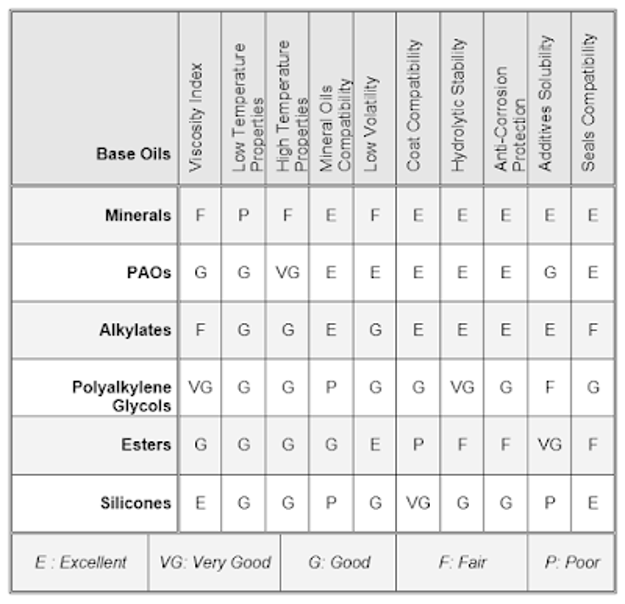
Their disadvantage is the formation of abrasive silicon oxides if oxidation does occur, effective adherent lubricating films are not formed due to their low surface tension, and they also show poor response to additives. They are used as brake fluids and as antifoam agents in lubricants. The table compares different synthetic base oils properties against mineral oil. Comparison among base oils.
Bio-bases Oils
They are mainly produced from soybeans, rapeseed, palm tree, sunflowers and safflowers. Their advantages are high biodegradability, superior lubricity, higher flash point and viscosity index; but their pour point is high and the oxidative stability is poor, also the recycling is difficult.
Main applications are hydraulic fluids, transmission fluids, gear oils, compressor oils and greases. Better when application is total loss, indoors or where low pour point is not an issue, food industry or environmentally-sensitive areas.
Additives
Lubricants require additional ingredients beyond a base oil to provide functionality. The following is a list of the common materials used. Additives 5% to 30% of an oils formula with engine oil using the highest concentration.
Typical passenger car engine oil contains detergents, dispersants, rust inhibitors, anti-wear additives, pour depressants, antioxidants, anti-foam additives and friction modifiers. Anti-wear additives help reduce wear between heavily loaded engine parts; detergents and dispersants help prevent buildup of contaminants, sludge, soot and varnish; and oxidation inhibitors help prevent lubricant breakdown at high operating temperatures.
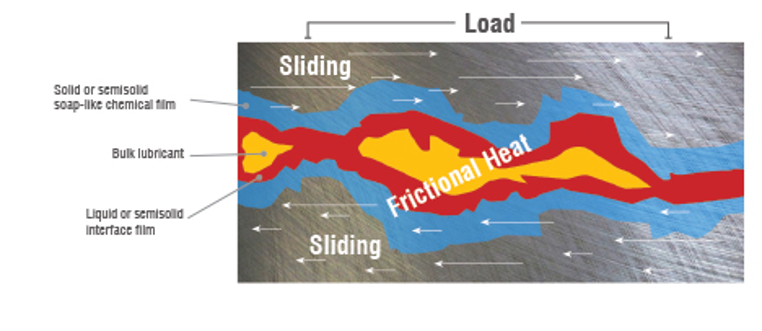
Extreme Pressure (EP) Agents – a phosphorus, sulfur, or chlorine-based additive typically used in gear oils that prevents sliding metal surfaces from seizing under conditions of extreme pressure. At high local temperatures it combines chemically with the metal to form a surface film. The EP additives made of sulfur, phosphorus, or chlorine. They become reactive at a high temperature (160+F) and will attack yellow surfaces and can be slightly corrosive to some metals, especially at elevated temperatures.
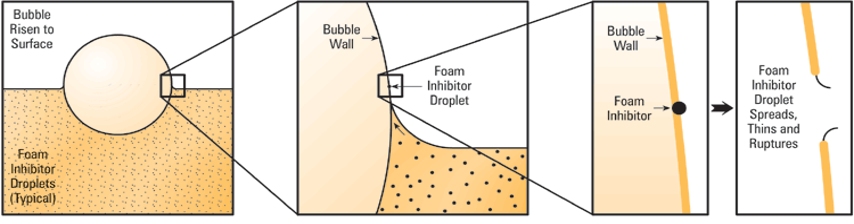
Antifoam or Foam Inhibitor – silicone-based additives used in turbulent systems, it helps combine small air bubbles into large bubbles which rise to the surface and burst. It decreases the surface tension of the bubble to thin and weakens it so that it pops. Most oils contain foam inhibitors that work by altering the surface tension of the oil. It allows bubbles to combine and break. Foam inhibitors are either based on silicone or are organic antifoam agents.
Rust and Corrosion Inhibitors – carbon-based molecules designed to absorb onto metal surfaces to prevent attack by air and water. Rusting and corrosion work by slowing the deterioration of a component surface due to a chemical attack by acidic products of oil oxidation. Rusting refers to the process of a ferrous surface oxidizing due to the presence of water in oil. Oils that contain rust and oxidation inhibitors are known as R&O oils in the US, and HL oils overseas.
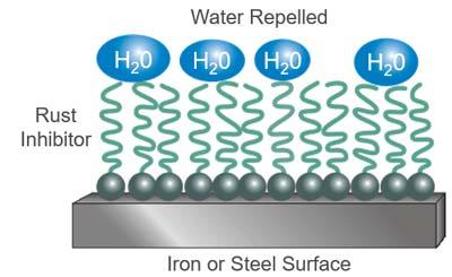
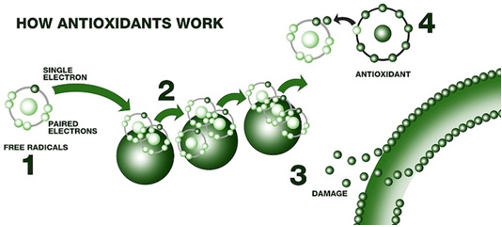
Oxidation Inhibitors – amine and phenolic antioxidants act by interrupting the free radical chain reaction that results in oxidation. Essentially, as the oil starts to decompose in the presence of oxygen, these inhibitors interrupt the reaction. They also keep metal from speeding up the oxidation reaction by deactivating the metal. Oxidation inhibitors are added to extend the life of the oil. Oxygen reacts with the oil to produce weak acids that can pit surfaces. Oxidation inhibitors slow the rate of oxidation.
Oxidation stability is important in most compressor applications because of the heat that is generated. Oxidized oil can create deposits that build up on discharge valves allowing them to stick open. This causes hot air to get sucked back into the compression chamber where it is recompressed. The air can generate enough heat to ignite the deposits and cause a fire or explosion. Use of synthetics can minimize this possibility.
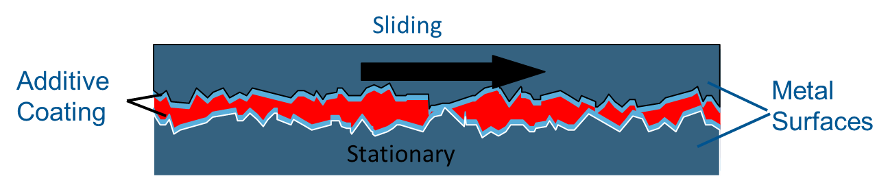
Anti-wear Additive – Zinc dialkyl dithiophosphate (ZDDP) is the most common anti-wear additive, although there are many zinc-free additives based on sulfur and phosphorus that also impart anti-wear properties. The zinc-sulfur-phosphorus end of the molecule is attracted to the metal surface allowing the long chains of carbons and hydrogens on the other end of the molecule to form a slippery carpet that prevents wear.
Not a chemical reaction, rather a super-strong attraction. There are other anti-wear additives that do not contain zinc. Some are based on sulfur, and some on fatty materials. Anti-wear additives, as a rule, are not as aggressive as extreme pressure additives. Oils that contain anti-wear additives are often called AW oils in the US or carry the HLP designation in Europe. Zinc containing anti-wear oils are generally not recommended for air compressors because the anti-wear package may compromise the oxidation stability of the oil.
Demulsifier – carbon-based polymers affect the interfacial tension of contaminants, so they separate out from oil rapidly. Hydrolytic stability is the ability of the oil to resist degradation in the presence of water. This is important because any system open to the atmosphere will be exposed to some moisture from humidity and condensation. Some ester-based fluids have relatively poor hydrolytic stability and will rapidly turn acidic in the presence of water.
Pour Point Depressants – chemicals designed to reduce the solidification of the oil to the lowest temperature at which it will pour under an ASTM laboratory test. Typically, these are methacrylate molecules and will inhibit the crystallization of the wax molecules.

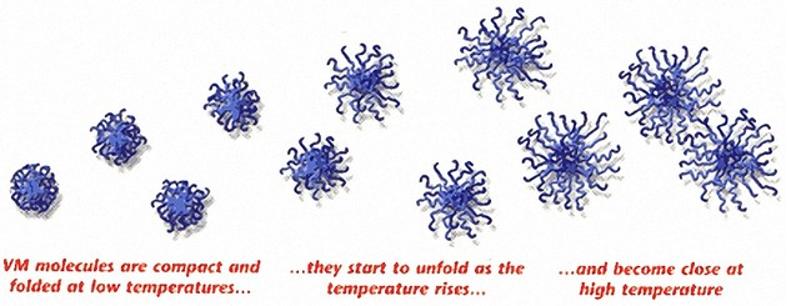
Viscosity Index Improvers – chemicals designed to reduce the thinning of an oil when the temperature increases. These chemicals are typically methacrylate molecules and will inhibit the thinning of the oil by expanding their molecular footprint this reducing flowability as the temperature increases.
Detergents – typically used in engine oil formulas, they are designed to keep the system clean of deposits. Often, they are alkaline by nature thus contribute to increase then TBN of the oil. Diesel engine lube oils are compounded with alkaline additives to help neutralize acids from combustion. They also provide antioxidant properties. Typical compounds contain calcium or magnesium.
Detergents have their disadvantages. Detergents move deposits downstream where they may build up on heat transfer surfaces in coolers. Detergent oils absorb water. If water can build up in the oil, it will cause rust and will accelerate oxidation. Compressors generate water because the humidity from the air condenses as the air is compressed. It is generally removed in a coalescer or knockout drum, but some water gets into the oil. For this reason, detergent oils are only used in limited applications.
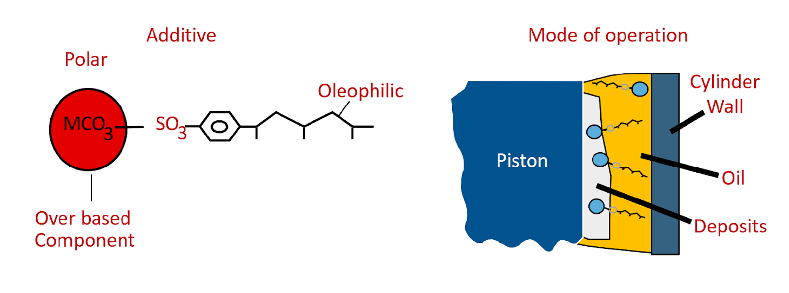
Dispersants – designed to capture particulates such as soot to form a micelle and keep in suspension. These compounds can be part of the detergent chemistry or be metal-free so they can be used in an ashless formulations. Some additives can actually contribute to wear. Too much metallic detergent/dispersant can leave ash type deposits that can be abrasive. There is a test to measure the amount of ash left behind when an oil is burned. It is commonly known as a sulfated ash test. Some engine manufacturers limit the amount of ash that is in an oil. An “ashless” oil required for some aviation engines has less than 0.1% ash, while a high ash oil used in some marine engines with high sulfur fuel can have ash in excess of 1.5%.
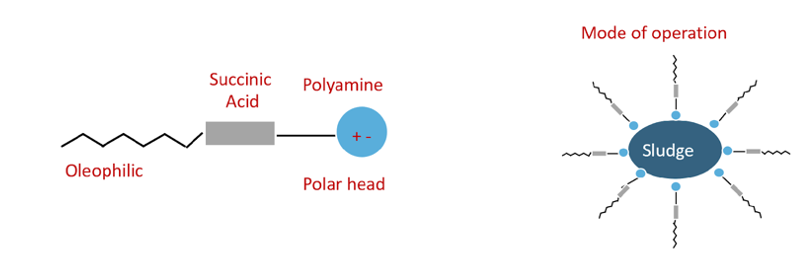
Additives can be depleted in service. There is a quick field test used to measure the level of detergency and dispersant of used oils. It is commonly known as the Oil spot (or patch) test. A simple test is when oil is filtered through a patch and treated with a solvent. If particles are concentrated in the center of the patch, it indicates that water or anti-freeze may be impairing dispersancy. The oil spot test can also pick up fuel soot, which are particles formed from fuel that is not completely burned. The filter patch can show evidence of dirt contamination, too.
Compatibility
Lubricant additives were developed to enhance the existing characteristics of the base oil(s) a lubricant is formulated with, to reduce the deficiencies of the base oils(s) or impart new performance characteristics. Engine oils were the first lubricants to be formulated with additives. They have been and still are the largest market segment for lubrication. So, it is no surprise that most of the research and development efforts have been placed on engine oil enhancement.
In 1911, the American Society of Automotive Engineers (SAE) established the oil classification system. This was related only to oil viscosity and not performance. Until the 1930s, engine oils did not contain any additives. They were only base oils. Prior to the introduction of additive chemistry, the oil drain intervals were 750 miles. Due to increasing consumer demands and economic pressures, internal combustion engines became more sophisticated. Engine oils were becoming increasingly stressed and challenges on their performance reserves gave rise to a need for additives.
The first oil additive developed was the pourpoint depressant. These acrylate polymers were developed in the mid-1930s. Anti-wear additives such as zinc dithiophosphate were introduced in the early 1940s followed by corrosion inhibitors and then sulfonate detergents. The sulfonate detergents were found to provide acid neutralization as well as oxidation inhabitation as well as rust and corrosion inhabitation.
In 1932, the American Petroleum Institute (API) established a specification system for engine oil performance classification. This is an important consideration because it is the only system by which a lubricant can be deemed compatible with another from a different manufacturer without the need to test compatibility. As long as the oils are of the same viscosity grade and have the same API classification and SAE viscosity, the oils are compatible; the user can mix oils if need be. This is not the case for other lubricants.
When mixing different lubricants, an adverse reaction may occur between two oils at certain working conditions in a system. This is considered ‘lubricant incompatibility’. Most often the cause of incompatibility is the neutralization of an acidic additive in one oil by an alkaline additive in the other oil. The result is that the additives react with each other instead of the metal surface, particle or free radicals in the oil.
The newly formed compound becomes ineffective and precipitate (drop out). Most all additives are polar which is what drives this reaction. This is by design. The polarity affords surface reaction as well as contamination reactions all that benefit the asset. During the reaction of incompatibility, often a soap forms that can precipitate a grease-like gel that interferes with lubrication and oil flow.
However, mixed oils may not always lead to incompatibility issues. They can exist without precipitation or reaction in an operating system for an indefinite period until water is introduced. Water can quickly lead to a reaction between the polar additives. Iron and copper found on the molecular level can act as catalysts in these reactions. Incompatibility reactions are not reversible. Removing water by drying the system and the oil does not remove the formed gel or eliminate the soap.
Typically, acidic additives can be found in gear, hydraulic and some circulating oils. Alkaline-based additives are used in engine oils. There are some additives that are neither acidic nor basic but neutral, these types of additives are used in compressors and refrigeration oils. Additives that are acidic are identified as being strong acids and will react faster than acids that are formed during the initiation stage of oxidation, which are typically carboxylic acids or nitric acids, and are weak acids due to the limited number to protons donated.
Weak acids react slower than strong acids. This is the reason why oils that have incompatible additive chemistry react so fast. Additives are not the only culprit. Propylene glycols, polyglycols, phosphate esters, polyol esters base oils have fair to poor compatibility with mineral oil-based lubricants. While these oils may not for solid substances, they may form a sludge. Many will not mix with the mineral-based lubricants.